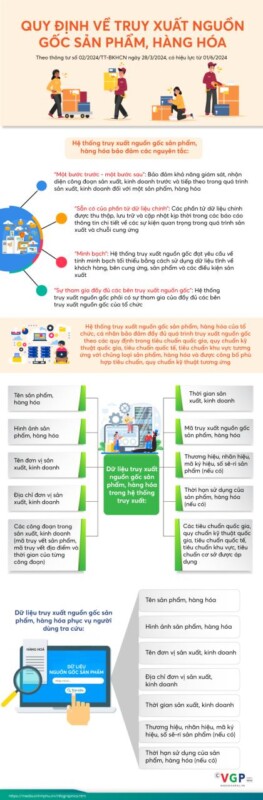
Israel thực hiện cải cách chế độ/chính sách nhập khẩu đối với các loại hàng hóa, tạo thuận lợi cho hoạt động ngoại thương và bãi bỏ các rào cản thương mại, nhằm góp phần làm giảm chi phí sinh hoạt đối với cuộc sống của người dân.
Văn bản 01: xem tại đây
Review of the New Import Reform in Israel
The Israeli parliament has recently approved a major reform, intended to facilitate trade
and to remove trade barriers. It is called “the import reform” and it includes two main
principles:
1. Products that comply with the regulation in major advanced countries should be good
for Israel as well.
2. The method of enforcement should be based on importers declaration alongside with
smart and effective marker surveillance.
This reform is implemented differently for different products and includes changes in the
regulation for food, cosmetics, electric home appliances, and many other products that
needs to comply with an official Israeli standard.
Naturally, the reform also requires a conceptual change of perspective by importers
regarding their responsibility over having the imported products uphold the requirements
set by local regulators and over their capability of supporting their declarations with
appropriate documentation as required in Israel. It is important to understand that
substantial enforcement powers were given for reacting to violations in these contexts,
including powers to impose financial sanctions of hundreds of thousands of Shekels.
Here is a short overlook of the reform:
Standardization – The major amendments to the Standards
Law as well as the Import and Export Ordinance
Official standards applies to many products, including: electrical and electronic products,
houseware, chemicals and toys (The reform does not apply to all products in the same
way).
This is a comprehensive reform that includes significant changes in two laws.
Two key issues underline the reform:
1. An extensive move to declaration-based control (instead of the need for an Israeli
laboratory approval).
Date of modification – June 2022 (with an option to defer execution by 9 months.)
In the coming weeks, the Ministry of Economy is expected to issue an Importation Group
Order that will switch the standards to Importation Group 2 (model approval + declaration
per shipment) or to Importation Group 3 (declaration per shipment.)
There are a limited number of standards that we already know will remain in the most
strict import regime (Group 1), which requires a full model approval + a check done by a
certified Israeli lab every shipment:
• Lifting equipment
• Pressure equipment
• Devices or appliances that are in use in liquefied petroleum gas cooking gas) systems,
including gas tanks, regulators, meters, valves, pipeline, gas consuming appliances;
2
• Fire safety devices as well as fire detection and extinguishing equipment;
• Concrete reinforcement iron;
• Children’s playground devices;
• Toys for children up to the age of 3;
• Pacifiers and bottle nipples;
• Pacifier holders;
• Baby feeding bottles and tableware;
• Low- and medium-voltage power lines.
(It was also agreed that solar water-heaters, thermostats and blade-sharpening devices will
remain in Importation Group 1 for another six months.)
2. International control track (Cassis track)
Time of change – June 2022 (with an option to defer execution by 9 months.)
A decision was made to establish an international track, in which it would be possible to
import products that comply with international standards that were adopted in Israel
within an official standard, even if they fail to comply with local official standardization,
excluding labeling of products as required by Israeli law and adjustment of products to the
Israeli power grid.
This track follows the last updated version of the standardization adopted in Israel, even if
the standardization authorities are yet to incorporate this up-to-date version in the
officially exercised standardization in Israel.
The standards specified in the third supplement to the law (which is a supplement that is
currently listing many dozens of standards and standard parts) were excluded from this
track, however, the list of exclusions is expected to be reduced in the future.
Concerning products that an official standard, which does not adopt foreign standards, is
applied to them, the Minister of Economy is empowered to directly adopt an international
standard that will enable importation in such a track.
In this track, in relation to standards in importation groups 2 and 3, the importer is
obligated to attach to the declaration a test report from a laboratory qualified by an entity
that is a member in the ILAC, attesting to compliance with the international standard that
was adopted (and its updates) + an obligatory declaration that the goods in the shipment
are identical to those, to which the test report relates + an obligation to complete the
requirements of labeling and adjustment to the power grid.
Exceptions:
• When the foreign regulation contains a requirement for a certificate issued by a
Conformity Assessment Body regarding compliance with foreign regulatory
requirements, this will also be required in Israel.
3
• For the sake of proving compliance of the goods with standards that deal with
documentation, labeling and recording (such as part 1 of SI 2302 – similar to the EU
CLP), the person in charge of standardization is given the authority to request that a
document substituting the inspection certificate to attest to compliance with the
requirements of foreign regulation.
The Ministry of Economy is expected to publish user-friendly information regarding the
documents required for different standards.
3. Declaration submission procedure and initial overseeing
The Import and Export Ordinance was updated in such a way that a declaration of
Importation Groups 2 + 3 +the international track will be done by the importer, using
a testing lab within Israel (an acknowledged lab that was announced to apply to this
standard or the Israeli Standards institute.)
According to the risk management system of the Ministry of Economy, importers will
randomly be required, in relation to some of the shipments, to send the product portfolio
for testing and carrying out lab tests on some of the shipments.
If they were required to transfer the product portfolio, they will receive a certificate
approving meeting the requirements of the commissioner right after sending it to
testing (and in parallel to the inspection of its content) – the shipment cannot be
released before this is done.
If they are required to put the product to testing, upon receiving the importer’s
commitment to transfer it to be tested, a certificate approving meeting the
requirements of the commissioner will be issued. The shipment cannot be released
before this is done.
If they are not required to do such tests, the importer will received within 3 days a
certificate approving meeting the requirements of the commissioner.
4. Requiring a product portfolio
We wish to remind that the requirement for a product portfolio has been around for
years and all the importers are required to ensure that they are familiar with and
upholding these requirements.
As part of the reform, the supplement was amended to better match the requirements
in the procedures of the standardization commissioner and those in the supplement to
the Importation and Export Ordinance and we believe that it is easier to implement.
The amendment made in this context entered effect upon their publication in the
Official Gazette. We are in contact with the standardization administration to ensure
that the manual they drew in context of the requirement of the product requirement
will be updated and we will hold with them a designated training on this matter.
Enforcement regarding requirement of a product portfolio is expected to be significantly
increased and boosted with many strict enforcement authorities, so it is important that
importers are prepared in accordance with the requirements.
5. Enforcement authorities
4
The Ministry of Economy has set as a condition to execute the reform (that offers the
importers a significant easement) acceptance of substantial and deterring enforcement
tools, including manpower quotas, budget for advanced IT systems, collaboration with
Customs and authority to impose hundreds of thousands or Shekels as financial
sanctions.
6. Cutting short the process of updating a standard
The Standards Law was amended for the sake of cutting short the timetables for
updating an official standard, allowing local standardization to react quicker to changes
in the adopted standards.
7. Product portfolio and labeling requirement easement – specific causes
Regarding products that are not designed to be marketed and distributed within the
public that are:
• Samples;
• A product intended to be used in production procedures;
• A product intended to be self-used;
• Industrial spare parts Those are exempt from the requirements of labeling within an
official standard and from maintaining a product portfolio.
8. Exemption from compliance with requirements of an official standard – export +
research and development.
The Standards Law was amended in a way that provides exemption from the official
standard requirements to:
• Manufacturing or importation of products that are intended for research or
development as those are defined in the Encouragement of Research, Development and
Innovation in Industry Law.
• Products that are intended for export.
9. Exceptions Committee
As part of the amendments to the varied laws that are part of the import reform, it was
decided to establish an Exceptions Committee, before which future proposals will be
presented, if such will be brought up, including application of stricter importation
requirements that apply to a variety of products. The Ministry of Economy is given an
authority to apply stricter requirements without requiring an approval of the
Committee for a limited period and only in urgent cases.
Energy efficiency reform – requirements for electric
appliances – Amendments to the Energy sources law.
Date of modification – September 2022 (with an option to defer execution by 9 months.)
Throughout the years, the Ministry of Energy has established in accordance with the
Energy Sources Law a list of regulations that refer to the requirements of energy
efficiency of a variety of electric appliances and electronic devices. As part of the import
5
reform, the law was amended in such a way that a direct reference was made from it to
the relevant European energy efficiency regulation (instead of those that exist nowadays
in the regulations) for the following products:
• Chillers;
• Air conditioners;
• Refrigerators;
• Washing machines;
• Tumble driers;
• Dishwashers;
• Ovens;
• Light bulbs;
• Ballast for fluorescent lamps;
• TV adapters;
• Televisions;
• Electric asynchronous three-phase induction motor cage rotors;
• Electric appliances with standby mode.
In addition, the law defines a declaration-based importation process for those products
and the declaration should be supported with either of the following:
1. A relevant DOC (declaration of conformity) document from the manufacturer with an
energy efficiency label (when the European regulation states that such a label is required.)
2. An inspection certificate from a qualified laboratory that was ordained by an entity that
is a member in ILAC, the Israeli Standards Institute or an authorized lab, according to
which the product complies with the energy efficiency level of the electric appliance as
mentioned in the relevant European regulation and will attach to it an energy rating label
complying with the electric appliance’s energy efficiency level if such a label is required
by the European regulation.
The importer must undertake that the appliance for which the certificate was issued is
identical to the one he imports.
The amendment of the law also regulates a list of products that are required to bear an
energy label and those that are not, and defined enforcement authorities.
The Chamber of Commerce requested the Ministry of Energy to establish regulations that
will allow transition periods, during which we believe that the currently existing
regulations should be allowed to be in effect alongside the adopted European regulation.
We have yet to receive a respond.
6
Food – review of the main amendments to the Protection of
Public Health Law (Food)
The Public Health Protection Law (food) was amended according to the following basic
principles. The reform is expected to include a number of updates for the importer:
o Adoption of EU regulation stating the requirements in Europe in terms of chemical and
biological contaminants (excluding listeria and salmonella) and pesticide residues (with
certain exceptions).
o Definition of “GIP – good importer practice” –this is a new definition for an importer
who has HACCP based procedures in regards to food quality and safety.
o New import route for the “GIP” – the “European Route” Enables the import of food
based on the declaration even for some of the products that are “sensitive” food products
(such as pasteurized dairy products; honey products; gelatin and collagen based products,
low-level acidity canned food; products that needs to be stored or delivered in a
temperature lower than 8◦C, drinking and mineral water in bottles, food coloring and
others).
o Removing trade barriers in Israeli food standards.
o The reform is expected to include a comprehensive enforcement plan with separate
memorandum to be approved later, with stringent criteria and the authority to impose
significant financial sanctions for violations.
Valid as from January 2023 with 9-month postponement option.
As part of the import reform, there is also a very significant reform in food regulation in
general and import requirements and procedures in particular; following is a review of the
main points of this part of the reform.
The essence of the Amendments:
1. Adoption of the European Union regulations – One of the toughest issues that the food
business operators face is the differences between the regulatory requirements in Israel
and those acceptable in other developed countries. For years, the chamber’s food sector
has been working towards cancellation of requirements so that the Israeli importer and his
supplier could speak the same language.
As part of the reform, our position was accepted and the law included a direct adoption of
the European regulatory requirements in aspects of chemical and biological contaminants.
(Excluding Listeria and Salmonella that will be established separately), residues of
mercury compounds and pesticides residues, with the legislative infrastructure offers a
future possibility to adopt additional European regulatory requirements. The law
determined that in case of a conflict between the adopted requirements and those
appearing in an official Israeli standard, the adopted ones will prevail.
Regarding raw meat, raw milk, fresh eggs in their shell and honey, the adopted European
requirements regarding contaminants and pesticide residues will not apply and in
7
addition, the European regulation of pesticide residues will not apply to fresh fruits and
vegetables.
Those issues will be discussed in an inter-ministerial, Health and Agriculture, team that is
expected to operate intensively in the coming months.
The binding version will be the English one and the Ministry of Health is also required to
present a Hebrew version for public review.
This part of the reform is scheduled to enter into force in January 2023, however, we are
working with the Ministry of Health in an attempt to facilitate an earlier implementation
of these adopted requirements as an alternative to the current regulation and to allow an
appropriate transition period, during which both requirements will be in effect in parallel.
The law also regulated a mechanism that is expected to enable swift and efficient
procedure of updates to the adopted European regulations to prevent emergence of gaps
between Israel and Europe. If the European regulations will be updated and the Ministry
of Health will opt to reject the update, the ministry will be required to go through an
Exceptions Committee, intended to make it difficult for the ministry to refrain from
adopting updates made in Europe.
2. ”Good Importer Practice” and the “European Track”:
A. Definition of a “good importer practice ” – This is a new definition of an importer that
will enable extending specific easements to importers who will comply with the included
requirements, thus promoting an increase of the professional level of importers and
reducing the workload imposed on National Food Service inspectors. Importers who wish
to do so should register as “good importer practice” at the Food Service. Applications to
be registered as a “good importer practice” – GIP can be submitted as of July 2022.
B. A new importation track designed for use of GIP – a declaration-based “European
Track” that will also apply to some of the food products that are defined as sensitive,
including pasteurized dairy products, honey and honey products, products that contain
either gelatin or collagen, low-acidity preserved food, food products that are defined as
sensitive only because they are required to be conveyed or stored in temperatures below 8
centigrade, mushrooms and mushroom mixtures, microorganisms for use in food
industries, bottled drinking and mineral water, food coloring For retail marketing.
What are the sensitive products that cannot be imported in the “European Track”?
Food for special medical purposes (FSMP)
Food supplements
Spirits
Non-pasteurized dairy products
Meat and derived products
Eggs and egg products
Food products that are intended to be consumed by infants and toddlers, including
formulas and supplementing food marked with such designation.
8
Winepress leaves Fish and fish products, including shellfish, crabs and echinoderms
C. It is noteworthy that strict requirements were defined in relation to importation of food
in the European Track, not just in relation to the importers, but also in reference to the
imported food and its producer; for instance, it is required:
Either of the following:
• A certificate attesting that the food production is overseen by an entity authorized to do
so in the country of production, issued by this competent entity (should be from the EU);
• Free trade certificate – this is relevant only for a free trade certificate deals with a sale
within the EU and issued by a competent European entity.
• Health certificate issued by an entity authorized to do so in the country of production
(should be from the EU);
• A certificate mentioned in article 52 (of the Protection of Public Health Law – Food),
attesting that the food was produced following Good Manufacturing Practice in the
country of production.
In addition, either of the following:
• Sale invoice from or to a European retailer;
• Shipping certificate to a European retailers;
• Free trade certificate issued by a competent authority within an EU country.
• In the matter of a manufacturer, for which the importer presented a health certificate
from a competent authority within the EU or one issued by a competent authority within
the EU overseeing the production of the food, there is another alternative, which is a food
producer declaration of compliance of the food with the EU regulatory requirements;
In addition, the third (b) annex to the law includes documents and details in the
application for a release certificate for food in the “European Track” and also has
individual requirements regarding a variety of products such as dairy food, low acidity
preserved food, mushrooms, products that contain gelatin, etc.
3. Handling unnecessary barrier in israeli food standards
This is a process that is expected to lead to revocation of a long line of local food
standards and articles in food standards.
Entering into effect – on January 1, 2023, with an optional deferment of up to 9 months.
4. Update of validity of a sensitive food import certificate to 6 years
As part of the attempt to reduce the extended waiting for sensitive food import
certificates, we have introduced into the version approved by the Economy Committee an
amendment that would allow extending the validity of a previously issued sensitive food
certificate from the currently issued 4 years to 6 years.
5. Application for releasing food shipments after leaving for Israel
9
Another amendment that should ease the importation process is the possibility to submit
an application to release a food shipment after this shipment left the country of origin to
Israel (instead of filing such an application close to the arrival of the shipment to Israel
and not a day earlier than the business day before the day of the shipment’s arrival.)
6. Obligating local food manufacturers to have a quality control systems by 2026
As part of the law, by 2026 all food producers in the State of Israel will be required to
have a quality control system. Regarding some of the foods, a stricter requirement of
GMP was defined: novel food, FSMP, food supplements, food marked as not containing
gluten, food intended to be consumed by infants and toddlers, including formulas and
supplementing food marked with such designation.
7. Enforcement
In order to counterbalance the easements, the reform is expected to include a
comprehensive enforcement program and a separate memorandum that is expected to be
approved later is supposed to define additional enforcement authorities. The enforcement
program is expected to include labor quotas and strict enforcement measures, including
the authority to impose substantial financial sanctions for violations.
8. Extending the temperature range for chilling unprocessed fresh meat
It was decided to amend article 9 of the Protection of Public Health Law – Food in context
of “fresh meat” in such a way that the temperature range in which unprocessed meat
would be chilled would be from (-1) to 4 oC. (Instead of 0 to 4 as it is presently).
Review of the main points of reform of importation of
cosmetic products as approved by the Knesset as part of the
Arrangements Law
As part of the importation chapter of the law, there is also a very significant reform in the
area of cosmetics; following is a review of the main points of the reform based on the
anticipated amendments to the Pharmacists Ordinance.
Upon importation of a cosmetic product, three alternative tracks will apply:
A. Importation based on compliance with the EU regulation – an importer would be
allowed to import a product that legally complies with the EU importation legitimacy
requirements and is legally marketed in at least one EU country; this will be achieved by
way of a notice regarding marketing that will be delivered on-line and will include
various details as specified by the law, such as manufacturer’s name, the cosmetic
product’s full Hebrew and English name, cosmetic product type, name, address and
contact details with the responsible person in charge, addresses of the cosmetic product’s
manufacturing sites, common name of either of the cosmetic product’s components,
photograph of the cosmetic product’s external and internal packaging from all sides in a
way that will allow identification of the cosmetic product, etc.
In addition, anyone choosing this alternative will not be allowed to market the cosmetic
product in Israel without the responsible person in charge appointed by the importer who
has made sure that all the aforementioned up-to-date documents and data in Hebrew and
10
English for the cosmetic product are in existence and without providing the cosmetic
product dealer a written approval of the existence of the aforesaid documents (hereinafter,
“the PIFProduct Information File”.)
• Photograph of the cosmetic product’s external and internal packaging from all sides in a
way that will allow identification of the cosmetic product;
• The cosmetic product’s safety assessment report;
• General description of the cosmetic product’s production method;
• Declaration of compliance with the standard listed in the fourth (a) supplement;
• Professional supporting evidence to the marketing statements related to the cosmetic
product in its cosmetic label, in accordance with the provisions set by the Minister of
Health;
• The cosmetic product’s manufacturer’s declaration that during the production process, as
it is defined in article 55h(f), there were no animal testing and if such did occur, data
regarding the performed testing, in accordance with the provisions set by the Minister of
Health or data regarding the performed testing, in accordance with the EU importation
legitimacy requirements.
It is clarified that the PIF will be available and accessible to the responsible person in
charge without delay; either digitally or in a hard copy in the responsible person’s Israeli
address as stated in the marketing notice, up to ten years after marketing the last batch of
the cosmetic product and that the responsible person in charge will ensure all the
following:
• The cosmetic product is manufactured under conditions that are required by the
provisions of the standard listed in the fourth (a) supplement;
• The cosmetic product is safe for use;
• The cosmetic product is effective for the purpose and true to the marketing statements it
is attributed in its cosmetic label;
In addition, the Product Information File (“PIF”) will be available without delay for
inspection by the administration for oversight and control over the cosmetic product, in
the responsible person’s Israeli address as stated in the marketing notice.
B. Parallel importation of a cosmetic products based on a certificate of compatibility to
the reference cosmetic product – if by December 31, 2022, the Minister did not establish
regulations regarding this alternative, the importer would be allowed to import in parallel
importation a cosmetic product listed in the fourth (b1) supplement, including after-shave,
aluminum-free roll-on deodorant, aluminum-free stick deodorant, compressed powder,
compressed blush, compressed shimmer, foot cream without Salicylic acid, (non-gel) nail
polish, leg hair removal wax, shampoo, conditioner, body soap, body cream, hand cream,
hair cream, excluding a sensitive cosmetic product, for as long as it was given a seal of
approval by any known lab and the importer informed the administration to this effect.
If the lab has found that, based on a sample of the reference cosmetic product and the
11
imported one, including their labels and other requirements by the law, the reference
cosmetic product and the imported one are identical; it will be allowed to be marketed.
C. Importation in accordance with the importation legality requirements that are currently
applicable and updated by the regulator from time to time. Importation if the existing
track will take place for at least 4 years in parallel with the option of importation based in
compliance with the EU regulation.
• Cancellation of tolls for cosmetics – the chairperson of the Economy Committee in the
Knesset, MK Michael Biton with agreement by the Ministry of Finance consented to our
request and an agreement was reached over canceling tolls for cosmetics registration. At
first stage, the toll will not apply to the notification track and later, the Control on
Commodities and Services Order (Cosmetics) 1973 will be amended by separate
legislation, leading also to cancellation of the registration tolls that are currently in effect.
This is intended to prevent discrimination between the tracks the importer has to choose
from.
• Entry into effect – On January 1, 2023, bearing in mind that the Minister of Health may
issue an order, with the ratification of the Knesset’s Labor, Welfare and Health
Committee, to defer this date by one period that will not exceed nine months, if the
Minister believes that the required preparation for commissioning the enforcement
authorities, supporting the provisions of the Pharmacists’ Ordinance, is yet to be
completed.
Remark: This is a general review, which is neither comprehensive nor binding, but merely
intended to help you understand the main topics of the reform. The mandatory documents
are those that were published in the OfficialGazett.
Source from FICC dated 1st June 2022
Văn bản 02: xem tại đây
Review of the New Import Reform in Israel
The Israeli parliament has recently approved a major reform, intended to facilitate trade
and to remove trade barriers. It is called “the import reform” and it includes two main
principles:
1. Products that comply with the regulation in major advanced countries should be good
for Israel as well.
2. The method of enforcement should be based on importers declaration alongside with
smart and effective marker surveillance.
This reform is implemented differently for different products and includes changes in the
regulation for food, cosmetics, electric home appliances, and many other products that
needs to comply with an official Israeli standard.
Naturally, the reform also requires a conceptual change of perspective by importers
regarding their responsibility over having the imported products uphold the requirements
set by local regulators and over their capability of supporting their declarations with
appropriate documentation as required in Israel. It is important to understand that
substantial enforcement powers were given for reacting to violations in these contexts,
including powers to impose financial sanctions of hundreds of thousands of Shekels.
Here is a short overlook of the reform:
Standardization – The major amendments to the Standards
Law as well as the Import and Export Ordinance
Official standards applies to many products, including: electrical and electronic products,
houseware, chemicals and toys (The reform does not apply to all products in the same
way).
This is a comprehensive reform that includes significant changes in two laws.
Two key issues underline the reform:
1. An extensive move to declaration-based control (instead of the need for an Israeli
laboratory approval).
Date of modification – June 2022 (with an option to defer execution by 9 months.)
In the coming weeks, the Ministry of Economy is expected to issue an Importation Group
Order that will switch the standards to Importation Group 2 (model approval + declaration
per shipment) or to Importation Group 3 (declaration per shipment.)
There are a limited number of standards that we already know will remain in the most
strict import regime (Group 1), which requires a full model approval + a check done by a
certified Israeli lab every shipment:
• Lifting equipment
• Pressure equipment
• Devices or appliances that are in use in liquefied petroleum gas cooking gas) systems,
including gas tanks, regulators, meters, valves, pipeline, gas consuming appliances;
2
• Fire safety devices as well as fire detection and extinguishing equipment;
• Concrete reinforcement iron;
• Children’s playground devices;
• Toys for children up to the age of 3;
• Pacifiers and bottle nipples;
• Pacifier holders;
• Baby feeding bottles and tableware;
• Low- and medium-voltage power lines.
(It was also agreed that solar water-heaters, thermostats and blade-sharpening devices will
remain in Importation Group 1 for another six months.)
2. International control track (Cassis track)
Time of change – June 2022 (with an option to defer execution by 9 months.)
A decision was made to establish an international track, in which it would be possible to
import products that comply with international standards that were adopted in Israel
within an official standard, even if they fail to comply with local official standardization,
excluding labeling of products as required by Israeli law and adjustment of products to the
Israeli power grid.
This track follows the last updated version of the standardization adopted in Israel, even if
the standardization authorities are yet to incorporate this up-to-date version in the
officially exercised standardization in Israel.
The standards specified in the third supplement to the law (which is a supplement that is
currently listing many dozens of standards and standard parts) were excluded from this
track, however, the list of exclusions is expected to be reduced in the future.
Concerning products that an official standard, which does not adopt foreign standards, is
applied to them, the Minister of Economy is empowered to directly adopt an international
standard that will enable importation in such a track.
In this track, in relation to standards in importation groups 2 and 3, the importer is
obligated to attach to the declaration a test report from a laboratory qualified by an entity
that is a member in the ILAC, attesting to compliance with the international standard that
was adopted (and its updates) + an obligatory declaration that the goods in the shipment
are identical to those, to which the test report relates + an obligation to complete the
requirements of labeling and adjustment to the power grid.
Exceptions:
• When the foreign regulation contains a requirement for a certificate issued by a
Conformity Assessment Body regarding compliance with foreign regulatory
requirements, this will also be required in Israel.
3
• For the sake of proving compliance of the goods with standards that deal with
documentation, labeling and recording (such as part 1 of SI 2302 – similar to the EU
CLP), the person in charge of standardization is given the authority to request that a
document substituting the inspection certificate to attest to compliance with the
requirements of foreign regulation.
The Ministry of Economy is expected to publish user-friendly information regarding the
documents required for different standards.
3. Declaration submission procedure and initial overseeing
The Import and Export Ordinance was updated in such a way that a declaration of
Importation Groups 2 + 3 +the international track will be done by the importer, using
a testing lab within Israel (an acknowledged lab that was announced to apply to this
standard or the Israeli Standards institute.)
According to the risk management system of the Ministry of Economy, importers will
randomly be required, in relation to some of the shipments, to send the product portfolio
for testing and carrying out lab tests on some of the shipments.
If they were required to transfer the product portfolio, they will receive a certificate
approving meeting the requirements of the commissioner right after sending it to
testing (and in parallel to the inspection of its content) – the shipment cannot be
released before this is done.
If they are required to put the product to testing, upon receiving the importer’s
commitment to transfer it to be tested, a certificate approving meeting the
requirements of the commissioner will be issued. The shipment cannot be released
before this is done.
If they are not required to do such tests, the importer will received within 3 days a
certificate approving meeting the requirements of the commissioner.
4. Requiring a product portfolio
We wish to remind that the requirement for a product portfolio has been around for
years and all the importers are required to ensure that they are familiar with and
upholding these requirements.
As part of the reform, the supplement was amended to better match the requirements
in the procedures of the standardization commissioner and those in the supplement to
the Importation and Export Ordinance and we believe that it is easier to implement.
The amendment made in this context entered effect upon their publication in the
Official Gazette. We are in contact with the standardization administration to ensure
that the manual they drew in context of the requirement of the product requirement
will be updated and we will hold with them a designated training on this matter.
Enforcement regarding requirement of a product portfolio is expected to be significantly
increased and boosted with many strict enforcement authorities, so it is important that
importers are prepared in accordance with the requirements.
5. Enforcement authorities
4
The Ministry of Economy has set as a condition to execute the reform (that offers the
importers a significant easement) acceptance of substantial and deterring enforcement
tools, including manpower quotas, budget for advanced IT systems, collaboration with
Customs and authority to impose hundreds of thousands or Shekels as financial
sanctions.
6. Cutting short the process of updating a standard
The Standards Law was amended for the sake of cutting short the timetables for
updating an official standard, allowing local standardization to react quicker to changes
in the adopted standards.
7. Product portfolio and labeling requirement easement – specific causes
Regarding products that are not designed to be marketed and distributed within the
public that are:
• Samples;
• A product intended to be used in production procedures;
• A product intended to be self-used;
• Industrial spare parts Those are exempt from the requirements of labeling within an
official standard and from maintaining a product portfolio.
8. Exemption from compliance with requirements of an official standard – export +
research and development.
The Standards Law was amended in a way that provides exemption from the official
standard requirements to:
• Manufacturing or importation of products that are intended for research or
development as those are defined in the Encouragement of Research, Development and
Innovation in Industry Law.
• Products that are intended for export.
9. Exceptions Committee
As part of the amendments to the varied laws that are part of the import reform, it was
decided to establish an Exceptions Committee, before which future proposals will be
presented, if such will be brought up, including application of stricter importation
requirements that apply to a variety of products. The Ministry of Economy is given an
authority to apply stricter requirements without requiring an approval of the
Committee for a limited period and only in urgent cases.
Energy efficiency reform – requirements for electric
appliances – Amendments to the Energy sources law.
Date of modification – September 2022 (with an option to defer execution by 9 months.)
Throughout the years, the Ministry of Energy has established in accordance with the
Energy Sources Law a list of regulations that refer to the requirements of energy
efficiency of a variety of electric appliances and electronic devices. As part of the import
5
reform, the law was amended in such a way that a direct reference was made from it to
the relevant European energy efficiency regulation (instead of those that exist nowadays
in the regulations) for the following products:
• Chillers;
• Air conditioners;
• Refrigerators;
• Washing machines;
• Tumble driers;
• Dishwashers;
• Ovens;
• Light bulbs;
• Ballast for fluorescent lamps;
• TV adapters;
• Televisions;
• Electric asynchronous three-phase induction motor cage rotors;
• Electric appliances with standby mode.
In addition, the law defines a declaration-based importation process for those products
and the declaration should be supported with either of the following:
1. A relevant DOC (declaration of conformity) document from the manufacturer with an
energy efficiency label (when the European regulation states that such a label is required.)
2. An inspection certificate from a qualified laboratory that was ordained by an entity that
is a member in ILAC, the Israeli Standards Institute or an authorized lab, according to
which the product complies with the energy efficiency level of the electric appliance as
mentioned in the relevant European regulation and will attach to it an energy rating label
complying with the electric appliance’s energy efficiency level if such a label is required
by the European regulation.
The importer must undertake that the appliance for which the certificate was issued is
identical to the one he imports.
The amendment of the law also regulates a list of products that are required to bear an
energy label and those that are not, and defined enforcement authorities.
The Chamber of Commerce requested the Ministry of Energy to establish regulations that
will allow transition periods, during which we believe that the currently existing
regulations should be allowed to be in effect alongside the adopted European regulation.
We have yet to receive a respond.
6
Food – review of the main amendments to the Protection of
Public Health Law (Food)
The Public Health Protection Law (food) was amended according to the following basic
principles. The reform is expected to include a number of updates for the importer:
o Adoption of EU regulation stating the requirements in Europe in terms of chemical and
biological contaminants (excluding listeria and salmonella) and pesticide residues (with
certain exceptions).
o Definition of “GIP – good importer practice” –this is a new definition for an importer
who has HACCP based procedures in regards to food quality and safety.
o New import route for the “GIP” – the “European Route” Enables the import of food
based on the declaration even for some of the products that are “sensitive” food products
(such as pasteurized dairy products; honey products; gelatin and collagen based products,
low-level acidity canned food; products that needs to be stored or delivered in a
temperature lower than 8◦C, drinking and mineral water in bottles, food coloring and
others).
o Removing trade barriers in Israeli food standards.
o The reform is expected to include a comprehensive enforcement plan with separate
memorandum to be approved later, with stringent criteria and the authority to impose
significant financial sanctions for violations.
Valid as from January 2023 with 9-month postponement option.
As part of the import reform, there is also a very significant reform in food regulation in
general and import requirements and procedures in particular; following is a review of the
main points of this part of the reform.
The essence of the Amendments:
1. Adoption of the European Union regulations – One of the toughest issues that the food
business operators face is the differences between the regulatory requirements in Israel
and those acceptable in other developed countries. For years, the chamber’s food sector
has been working towards cancellation of requirements so that the Israeli importer and his
supplier could speak the same language.
As part of the reform, our position was accepted and the law included a direct adoption of
the European regulatory requirements in aspects of chemical and biological contaminants.
(Excluding Listeria and Salmonella that will be established separately), residues of
mercury compounds and pesticides residues, with the legislative infrastructure offers a
future possibility to adopt additional European regulatory requirements. The law
determined that in case of a conflict between the adopted requirements and those
appearing in an official Israeli standard, the adopted ones will prevail.
Regarding raw meat, raw milk, fresh eggs in their shell and honey, the adopted European
requirements regarding contaminants and pesticide residues will not apply and in
7
addition, the European regulation of pesticide residues will not apply to fresh fruits and
vegetables.
Those issues will be discussed in an inter-ministerial, Health and Agriculture, team that is
expected to operate intensively in the coming months.
The binding version will be the English one and the Ministry of Health is also required to
present a Hebrew version for public review.
This part of the reform is scheduled to enter into force in January 2023, however, we are
working with the Ministry of Health in an attempt to facilitate an earlier implementation
of these adopted requirements as an alternative to the current regulation and to allow an
appropriate transition period, during which both requirements will be in effect in parallel.
The law also regulated a mechanism that is expected to enable swift and efficient
procedure of updates to the adopted European regulations to prevent emergence of gaps
between Israel and Europe. If the European regulations will be updated and the Ministry
of Health will opt to reject the update, the ministry will be required to go through an
Exceptions Committee, intended to make it difficult for the ministry to refrain from
adopting updates made in Europe.
2. ”Good Importer Practice” and the “European Track”:
A. Definition of a “good importer practice ” – This is a new definition of an importer that
will enable extending specific easements to importers who will comply with the included
requirements, thus promoting an increase of the professional level of importers and
reducing the workload imposed on National Food Service inspectors. Importers who wish
to do so should register as “good importer practice” at the Food Service. Applications to
be registered as a “good importer practice” – GIP can be submitted as of July 2022.
B. A new importation track designed for use of GIP – a declaration-based “European
Track” that will also apply to some of the food products that are defined as sensitive,
including pasteurized dairy products, honey and honey products, products that contain
either gelatin or collagen, low-acidity preserved food, food products that are defined as
sensitive only because they are required to be conveyed or stored in temperatures below 8
centigrade, mushrooms and mushroom mixtures, microorganisms for use in food
industries, bottled drinking and mineral water, food coloring For retail marketing.
What are the sensitive products that cannot be imported in the “European Track”?
Food for special medical purposes (FSMP)
Food supplements
Spirits
Non-pasteurized dairy products
Meat and derived products
Eggs and egg products
Food products that are intended to be consumed by infants and toddlers, including
formulas and supplementing food marked with such designation.
8
Winepress leaves Fish and fish products, including shellfish, crabs and echinoderms
C. It is noteworthy that strict requirements were defined in relation to importation of food
in the European Track, not just in relation to the importers, but also in reference to the
imported food and its producer; for instance, it is required:
Either of the following:
• A certificate attesting that the food production is overseen by an entity authorized to do
so in the country of production, issued by this competent entity (should be from the EU);
• Free trade certificate – this is relevant only for a free trade certificate deals with a sale
within the EU and issued by a competent European entity.
• Health certificate issued by an entity authorized to do so in the country of production
(should be from the EU);
• A certificate mentioned in article 52 (of the Protection of Public Health Law – Food),
attesting that the food was produced following Good Manufacturing Practice in the
country of production.
In addition, either of the following:
• Sale invoice from or to a European retailer;
• Shipping certificate to a European retailers;
• Free trade certificate issued by a competent authority within an EU country.
• In the matter of a manufacturer, for which the importer presented a health certificate
from a competent authority within the EU or one issued by a competent authority within
the EU overseeing the production of the food, there is another alternative, which is a food
producer declaration of compliance of the food with the EU regulatory requirements;
In addition, the third (b) annex to the law includes documents and details in the
application for a release certificate for food in the “European Track” and also has
individual requirements regarding a variety of products such as dairy food, low acidity
preserved food, mushrooms, products that contain gelatin, etc.
3. Handling unnecessary barrier in israeli food standards
This is a process that is expected to lead to revocation of a long line of local food
standards and articles in food standards.
Entering into effect – on January 1, 2023, with an optional deferment of up to 9 months.
4. Update of validity of a sensitive food import certificate to 6 years
As part of the attempt to reduce the extended waiting for sensitive food import
certificates, we have introduced into the version approved by the Economy Committee an
amendment that would allow extending the validity of a previously issued sensitive food
certificate from the currently issued 4 years to 6 years.
5. Application for releasing food shipments after leaving for Israel
9
Another amendment that should ease the importation process is the possibility to submit
an application to release a food shipment after this shipment left the country of origin to
Israel (instead of filing such an application close to the arrival of the shipment to Israel
and not a day earlier than the business day before the day of the shipment’s arrival.)
6. Obligating local food manufacturers to have a quality control systems by 2026
As part of the law, by 2026 all food producers in the State of Israel will be required to
have a quality control system. Regarding some of the foods, a stricter requirement of
GMP was defined: novel food, FSMP, food supplements, food marked as not containing
gluten, food intended to be consumed by infants and toddlers, including formulas and
supplementing food marked with such designation.
7. Enforcement
In order to counterbalance the easements, the reform is expected to include a
comprehensive enforcement program and a separate memorandum that is expected to be
approved later is supposed to define additional enforcement authorities. The enforcement
program is expected to include labor quotas and strict enforcement measures, including
the authority to impose substantial financial sanctions for violations.
8. Extending the temperature range for chilling unprocessed fresh meat
It was decided to amend article 9 of the Protection of Public Health Law – Food in context
of “fresh meat” in such a way that the temperature range in which unprocessed meat
would be chilled would be from (-1) to 4 oC. (Instead of 0 to 4 as it is presently).
Review of the main points of reform of importation of
cosmetic products as approved by the Knesset as part of the
Arrangements Law
As part of the importation chapter of the law, there is also a very significant reform in the
area of cosmetics; following is a review of the main points of the reform based on the
anticipated amendments to the Pharmacists Ordinance.
Upon importation of a cosmetic product, three alternative tracks will apply:
A. Importation based on compliance with the EU regulation – an importer would be
allowed to import a product that legally complies with the EU importation legitimacy
requirements and is legally marketed in at least one EU country; this will be achieved by
way of a notice regarding marketing that will be delivered on-line and will include
various details as specified by the law, such as manufacturer’s name, the cosmetic
product’s full Hebrew and English name, cosmetic product type, name, address and
contact details with the responsible person in charge, addresses of the cosmetic product’s
manufacturing sites, common name of either of the cosmetic product’s components,
photograph of the cosmetic product’s external and internal packaging from all sides in a
way that will allow identification of the cosmetic product, etc.
In addition, anyone choosing this alternative will not be allowed to market the cosmetic
product in Israel without the responsible person in charge appointed by the importer who
has made sure that all the aforementioned up-to-date documents and data in Hebrew and
10
English for the cosmetic product are in existence and without providing the cosmetic
product dealer a written approval of the existence of the aforesaid documents (hereinafter,
“the PIFProduct Information File”.)
• Photograph of the cosmetic product’s external and internal packaging from all sides in a
way that will allow identification of the cosmetic product;
• The cosmetic product’s safety assessment report;
• General description of the cosmetic product’s production method;
• Declaration of compliance with the standard listed in the fourth (a) supplement;
• Professional supporting evidence to the marketing statements related to the cosmetic
product in its cosmetic label, in accordance with the provisions set by the Minister of
Health;
• The cosmetic product’s manufacturer’s declaration that during the production process, as
it is defined in article 55h(f), there were no animal testing and if such did occur, data
regarding the performed testing, in accordance with the provisions set by the Minister of
Health or data regarding the performed testing, in accordance with the EU importation
legitimacy requirements.
It is clarified that the PIF will be available and accessible to the responsible person in
charge without delay; either digitally or in a hard copy in the responsible person’s Israeli
address as stated in the marketing notice, up to ten years after marketing the last batch of
the cosmetic product and that the responsible person in charge will ensure all the
following:
• The cosmetic product is manufactured under conditions that are required by the
provisions of the standard listed in the fourth (a) supplement;
• The cosmetic product is safe for use;
• The cosmetic product is effective for the purpose and true to the marketing statements it
is attributed in its cosmetic label;
In addition, the Product Information File (“PIF”) will be available without delay for
inspection by the administration for oversight and control over the cosmetic product, in
the responsible person’s Israeli address as stated in the marketing notice.
B. Parallel importation of a cosmetic products based on a certificate of compatibility to
the reference cosmetic product – if by December 31, 2022, the Minister did not establish
regulations regarding this alternative, the importer would be allowed to import in parallel
importation a cosmetic product listed in the fourth (b1) supplement, including after-shave,
aluminum-free roll-on deodorant, aluminum-free stick deodorant, compressed powder,
compressed blush, compressed shimmer, foot cream without Salicylic acid, (non-gel) nail
polish, leg hair removal wax, shampoo, conditioner, body soap, body cream, hand cream,
hair cream, excluding a sensitive cosmetic product, for as long as it was given a seal of
approval by any known lab and the importer informed the administration to this effect.
If the lab has found that, based on a sample of the reference cosmetic product and the
11
imported one, including their labels and other requirements by the law, the reference
cosmetic product and the imported one are identical; it will be allowed to be marketed.
C. Importation in accordance with the importation legality requirements that are currently
applicable and updated by the regulator from time to time. Importation if the existing
track will take place for at least 4 years in parallel with the option of importation based in
compliance with the EU regulation.
• Cancellation of tolls for cosmetics – the chairperson of the Economy Committee in the
Knesset, MK Michael Biton with agreement by the Ministry of Finance consented to our
request and an agreement was reached over canceling tolls for cosmetics registration. At
first stage, the toll will not apply to the notification track and later, the Control on
Commodities and Services Order (Cosmetics) 1973 will be amended by separate
legislation, leading also to cancellation of the registration tolls that are currently in effect.
This is intended to prevent discrimination between the tracks the importer has to choose
from.
• Entry into effect – On January 1, 2023, bearing in mind that the Minister of Health may
issue an order, with the ratification of the Knesset’s Labor, Welfare and Health
Committee, to defer this date by one period that will not exceed nine months, if the
Minister believes that the required preparation for commissioning the enforcement
authorities, supporting the provisions of the Pharmacists’ Ordinance, is yet to be
completed.
Remark: This is a general review, which is neither comprehensive nor binding, but merely
intended to help you understand the main topics of the reform. The mandatory documents
are those that were published in the OfficialGazett.
Source from FICC dated 1st June 2022
Thương vụ Việt Nam tại Israel